
The Chehimi family: Zahra with daughter Lara (left) and Hussein with son Nehme. Zahra was paralyzed from the chest down as a result of a car accident in May 2013. (Photo: Chehimi family)
When Zahra Chehimi was paralyzed from the chest down by a car accident in May 2013, the 40-year-old mother of two was told she had little hope of recovery.
Despite her best efforts in a rehabilitation program at the Lyndhurst Centre of the Toronto Rehabilitation Institute, her spine had been fractured in several places and doctors agreed it was unlikely she would recover any mobility.
The mother was confined to a wheelchair and dependent on her husband to help her accomplish the day to day tasks that many take for granted.
"She doesn't feel normal anymore," said her husband, Hussein. "She isn't able to do things with our children the way she used to, which has been a big adjustment for our family."
But a clinical trial led by Dr. Michael Fehlings, Medical Director of the Krembil Neuroscience Centre, Head of the Spinal Program at Toronto Western Hospital and Senior Scientist, McEwen Centre for Regenerative Medicine, is giving the Chehimi family some hope that Zahra's prognosis might change in the future.
First neural stem cell trial in Canada
Toronto Western Hospital is one of three sites participating in an international clinical trial studying whether human neural stem cells can effectively treat spinal cord injury patients.
It is the first stem cell trial of its kind in Canada.
"We are pleased to be working at the forefront of this trailblazing study," said Fehlings. "There is a strong rationale to explore new therapeutic approaches, such as stem cells, for treating spinal cord injury in order to address the unmet medical need for new treatments to treat this devastating injury."
Chehimi was the first patient of the trial to be treated at Toronto Western.
On February 5, Chehimi underwent a surgical procedure to have the stem cells implanted in her spinal cord. She received four microinjections of approximately 20 million cells each, two above and two below the injury site in her spine.
The stem cells, labelled HuCNS-SC, are cultivated and provided by StemCells Inc., a company engaged in the research, development, and commercialization of cell-based therapeutics for use in stem cell-based research and drug discovery.
Spinal cord injury and the healing potential of stem cells
The theory behind the use of stem cells is: Axons are the primary transmission lines of the nervous system, relaying signals that connect the brain and spinal cord to the muscles and organs of the body. These nerve fibres run along the length of the spinal cord and transmit information to and from the brain, allowing for proper body function.
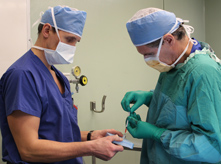
Dr. Michael Fehlings (right) prepares the first stem cell injection, one of four that he will administer. Each microinjection contains approximately 20 million cells. (Photo: UHN)
Axons are like electrical wires and are completely surrounded by myelin, a coating formed by specialized cells called oligodendrocytes. Myelin is essential for the proper functioning of the nervous system. In spinal cord injury, axons that survive the initial damage often lose this essential myelin coating due to death of oligodendrocytes. Axons that lose the myelin insulation do not carry the signals through the spinal cord properly and might eventually die. Axonal death and dysfunction lead to difficulties in movement and possible paralysis.
Research, including previous studies conducted in Fehlings' lab, has shown that when certain types of stem cells are injected into the spinal cord, they can take cues from the injured tissue which allow them to migrate and integrate properly to aid in repair. The injected stem cells are able to turn into oligodendrocytes and form myelin around the damaged axons, restoring the insulation and proper functioning of axons. This restored myelin allows for increased functional recovery following spinal cord injury.
The trial, which has shown promising results in patients treated in Switzerland, will further investigate the safety and efficacy of these stem cells to repair the damaged spinal cord. Researchers will follow up with patients at regular intervals for a year to track the effectiveness of the stem cells in their recovery.
Still early stages
In addition to this trial, different therapies are being evaluated and developed to address the different phases of spinal cord injury. It is hoped that the stem cell injection will not only contribute to the regeneration of the spinal cord, but also serve as a complement to other therapies.
However, although researchers obviously hope this trial will demonstrate that stem cells can encourage dramatic improvement in the mobility of its participants, it is also possible that patients might experience little to no improvement at all.
Despite this reality, Chehimi refused to be deterred. She remains optimistic she will regain some mobility, but also sees this as an opportunity to contribute to medical research.
"By being part of this research, I hope to open the door for other patients by helping experts discover a new treatment for spinal cord injury," she said.
Related Links
